Methods of Preparation of Alkynes
Methods of Preparation of Alkynes: Overview
This topic covers concepts, such as, Preparation of Alkynes, Preparation of Alkynes by Vicinal Dihalides & Preparation of Alkynes from Calcium Carbide etc.
Important Questions on Methods of Preparation of Alkynes
The reagent(s) for the following conversion,
is/are
The major product of the following reaction is
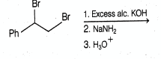
Which of the following reacting substances will not liberate ethyne gas?
Which of the following reacting substances will not liberate ethyne gas?
Which of the following reaction do not give alkyne :-
Phosphine, Acetylene and Ammonia can be formed by treating water with :-
Identify the compounds A and B in the following reaction sequence.
(s) + (l) A (g) B (l)
Identify the compound B in the reaction given below.
What will be the product formed when is treated with ?
Hydrolysis of which of the following carbides produce propyne?
On reaction with water, a metal carbide gives a colourless gas which burns readily in air. This gas, when passed through an ammoniacal silver nitrate solution, forms a precipitate. Identify the evolved gas.
A colourless gas is produced when metallic carbide undergoes treatment with water, which burns readily in air and gives a precipitate with ammoniacal silver nitrate solution. The gas evolved is:
When is treated with the product is formed. What will be the structure of
A metallic carbide on reaction with water gives a colourless gas, which burns readily in air and gives a precipitate with ammonical silver nitrate solution. What is the gas evolved in reaction?
Calculate the weight of precipitate formed, when grams of vicinal tetra bromopropane reacted with to form alkyne, which then passes through ammonical solution.
Which of the following reactions yields ethyne gas?
In which of the following reactions, acetylene gas will not be liberated?
When dibromoethane reacts with sodamide giving a product . The hybridisation state of the carbons present in it respectively, are
